Dimethyl Ether Market Size World Report, 2024
페이지 정보
작성자 Lawerence 날짜24-11-25 22:38 조회2회 댓글0건본문
China's consistently growing car market can also be anticipated to increase the dimethyl ether demand over the forecast interval. Furthermore, the simple availability of coal reserves in China will benefit the Asia Pacific regional dimethyl ether market. Furthermore, particular environmental laws set by most governments across the region will positively impression dimethyl ether improvement. In March 2021, SHV Vitality and KEW Know-how entered a joint enterprise often called Circular Fuels Ltd. This venture intended to develop renewable dimethyl ether (rDME) production plants and convert renewable and recycled carbon feedstock into renewable liquid fuel. Dimethylether and ethyl methyl ether are gases at atypical temperature. The other decrease homologues are colorless, nice smelling, risky liquids with typical ether smell. The C - O bonds in ether are polar and thus ethers have a web dipole moment. The weak polarity of ethers do not appreciably have an effect on their boiling factors which are comparable to these of the alkenes of comparable molecular mass.
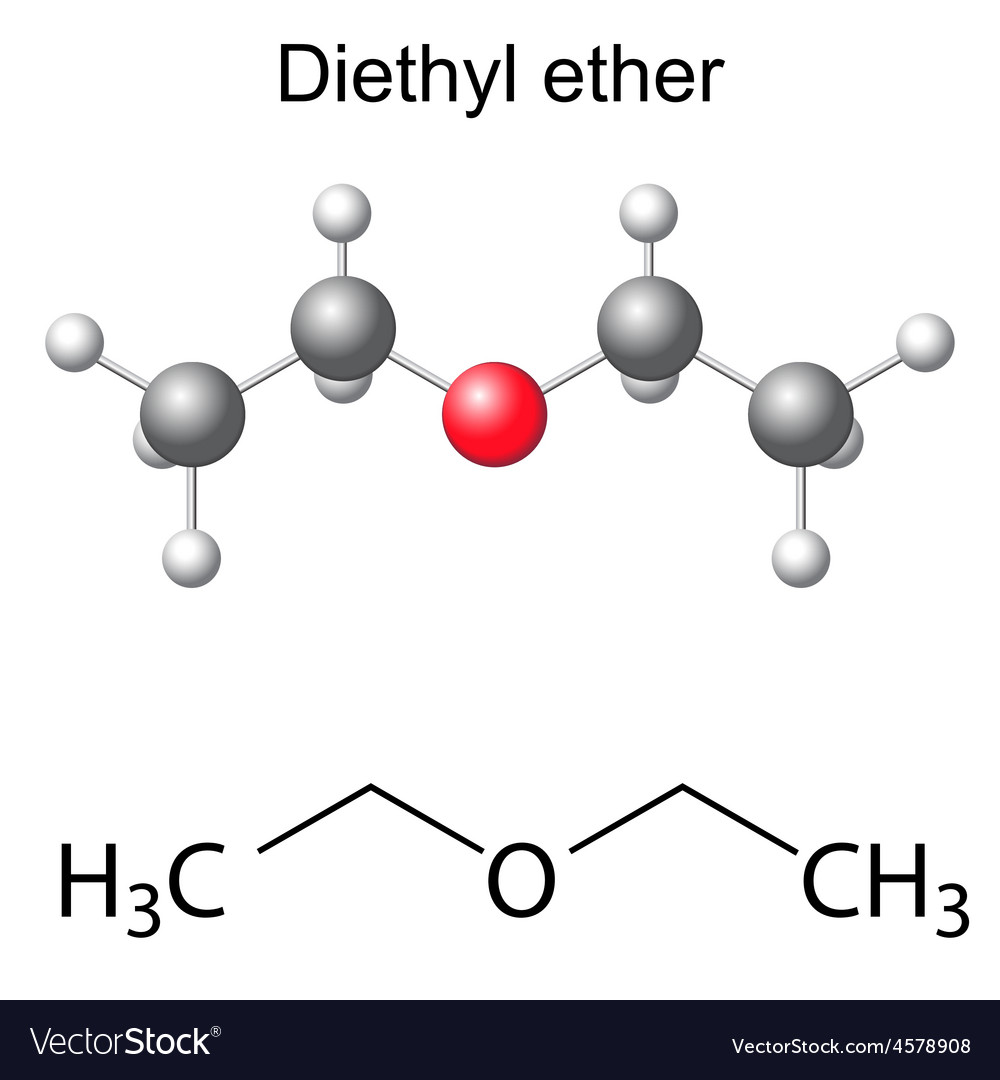
Dimethyl ether (DME) is a chemical compound with the formulation CH3OCH3. It is a colorless, flammable gas that is often used as a gas or solvent. DME is a clean-burning, low-carbon various to fossil fuels such as gasoline, diesel, and natural fuel, and it has a high octane quantity, which makes it appropriate to be used in inside combustion engines. Primarily based on the tip-users, the Dimethyl Ether Market is categorized Automotive, Pharmaceuticals, Agrochemicals and Others. In 2021, the Automotivesegment accounted for the most important share of the Market, with 39.6% and a Market revenue of 1.66 Billion. Throughout the forecast period, theAutomotivesegment is anticipated to maintain its main position while increasing at the quickest CAGRdue to a progress in the utilization of diethyl ether as a fuel additive in vehicles.
Get the newest insights on price motion and pattern evaluation of Dimethyl Ether (DME) in several areas internationally (Asia, Europe, North America, Latin America, and the Center East & Africa). Dimethyl ether prices had been found to be fluctuating for nearly all of the primary half of 2024. Upstream market dynamics primarily drove the price pattern for DME throughout the involved timeframe. The price trajectory of DME showed a stable development for many of the primary quarter. There was an oversupply state of affairs available in the market however, the demand was average which was unable to affect the value development in a very optimistic manner. 2-four), below Fourier Transform Ion Cyclotron Resonance Situations, J. Phys. Larson, J.W.; McMahon, T.B., Formation, Thermochemistry, and Relative Stabilities of Proton - Bound dimers of Oxygen n - Donor Bases from Ion Cyclotron Resonance Solvent - Trade Equilibria Measurements, J. Am. Lias, S.G.; Liebman, J.F.; Levin, R.D., Evaluated gasoline part basicities and proton affinities of molecules heats of formation of protonated molecules, J. Phys.
Thus, the water tends to proceed to have interaction in hydrogen bonding interactions with different molecules of its personal variety, and little or no is gained in terms of latest biphenyl-water interactions. Water is a terrible solvent for nonpolar hydrocarbon molecules: they're very hydrophobic (water-fearing). Next, you attempt a collection of increasingly giant alcohol compounds, starting with methanol (1 carbon) and ending with octanol (eight carbons). You find that the smaller alcohols - methanol, ethanol, and propanol - dissolve easily in water, at any water/alcohol ratio that you just strive. The plant now has an annual manufacturing capability of 1 million tons of methanol and 20,000 tons of DME, fuelled by natural fuel sourced from the islands. There's growing focus on decarbonisation of transport across the world. For example, in 2021, the U.S. Division of Power (DOE) Vehicle Applied sciences Office (VTO) granted over USD four million to fund testing of new purposes of dimethyl ether as a low-carbon replacement to fossil fuels. Oberon Fuels was a supporting companion.
Any leak can be either liquid or vapor. Dimethyl ether can asphyxiate by the displacement of air. Under extended publicity to fire or intense heat the containers might rupture violently and rocket. Highly flammable. Upon standing and exposure to air (oxygen) tendency to kind explosive peroxides. [Lewis, 3rd ed., 1993, p. Dimethyl ether is a colorless, extremely flammable gasoline (b. Very harmful fire and explosion hazard when exposed to flame, sparks, heat or robust oxidizers. Violent reaction with aluminum hydride, lithium aluminum hydride. Upon standing and publicity to air (oxygen) tendency to type explosive peroxides. In this course of, pure fuel What is dimethyl ether used for? combined with steam and uncovered to high temperatures and pressures in the presence of a catalyst. This reaction produces a mixture of hydrogen and carbon monoxide, which is then converted to DME through a course of known as fuel-shift. DME may also be produced from coal through a course of referred to as coal gasification. In this process, coal is exposed to excessive temperatures and pressures within the presence of oxygen, steam, and a catalyst. This reaction produces a mixture of hydrogen, carbon monoxide, and different gases, which might then be transformed to DME by a process just like the one used in the production of DME from pure gasoline.
댓글목록
등록된 댓글이 없습니다.















 광송무역
광송무역
 070-7762-8494
070-7762-8494